
G protein Signaling Regulation in Health and Disease
The overall research focus of my lab is to understand the integration and regulation of cell signaling mechanisms that facilitate the physiological and pathophysiological effects of various hormones, neurotransmitters and mechanical stimuli in the cardiovascular and renal systems. Specifically, the Osei-Owusu lab studies the regulation of signaling via heterotrimeric G proteins, which are mostly activated by G protein coupled receptors (GPCRs) and tightly controlled by GTPase activating proteins, including RGS proteins. Abnormal GPCR signaling is implicated in several human diseases and disorders such as depression, heart failure, chronic kidney disease, diabetes, stroke, and hypertension. In fact, more than half of all pharmacological agents for human diseases and disorders act on GPCRs to elicit their therapeutic effects. Therefore, studying how GPCR signaling is regulated in health and disease will lead to a better understanding of normal physiology and the pathogenesis of cardiovascular and renal diseases. Such knowledge is extremely important to improving health education, identifying novel drug targets, and developing new drugs or improving existing treatment regimens.
The Osei-Owusu Lab and its collaborators currently pursue several translational projects focused on pregnancy-related complications, hypertension, chronic kidney disease, and spinal cord injury. The main research approaches of the lab are pharmacology and integrative physiology, both of which involve functioning systems of molecules, cells, tissues, organs, and genetically engineered animals that recapitulate human diseases. These tools enable the lab to focus on whole-body function as it relates to human health and disease.
Dual Loss of RGS2 & 5 and Cardiac Arrhythmia
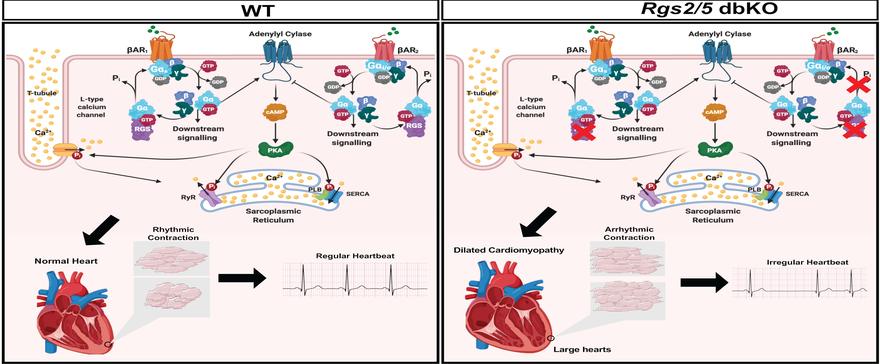
The regulation of cardiomyocyte contractility is a typical example of the lab’s focus on the relevance of G protein signaling regulation by RGS proteins in excitation-contraction coupling mechanisms in organ systems of the general circulation. For every heartbeat, each cardiac muscle cell receives electrical impulse initiated and propagated from the sinoatrial node for membrane depolarization to trigger calcium influx and additional calcium release from internal stores, that together initiate contraction. The rate and intensity of the contraction is moderated by catecholamines that act through G protein-coupled b-adrenergic receptors. RGS proteins fine-tune these signaling cascades to maintain normal cardiac rhythm. The loss of cardiac G protein signaling by RGS2 and 5 are implicated in cardiac arrhythmia, a major cause of cardiac death. Our lab is interested in understanding how RGS proteins are involved maintaining normal rhythm and how their absence leads to arrhythmia and heart failure.
RGS PROTEINS AND RENAL MECHANISMS OF HYPERTENSION
A major focus of the lab is the identification of novel mechanisms by which GTPase activating proteins (GAPs), specifically regulators of G protein signaling (RGS) proteins, control G protein signaling in the kidney to maintain normal blood pressure. Among more than 30 mammalian RGS proteins discovered to date, members of the B/R4 RGS protein family (including RGS1, 2, 4, 5, 8, 13, 16, 18, and 21) have been shown to play critical roles in cardiovascular and renal physiology. Understanding the function of RGS2 in renal mechanisms of blood pressure control is of particular interest to the lab, because mutations leading to decreases in the expression and/or function of this RGS protein is linked to human hypertension and other cardiovascular disorders. We use integrative physiology approach including mouse genetics, state-of-the-art radiotelemetry blood pressure monitoring, ex vivo vessel studies, immunohistochemistry, and cell and molecular biology methods to examine how the absence or mutations in the Rgs2 gene alter renal function and contribute to the pathogenesis of hypertension.
VASCULAR TONE REGULATION AND UTERINE BLOOD FLOW
Vascular tone of small-caliber arteries is a critical component of the primary determinants of arterial blood pressure and organ blood flow. Altered vascular tone of small resistance arteries in various vascular beds is a key feature and a hallmark of vascular remodeling that occurs in physiologic adaptation of the cardiovascular system during normal pregnancy, and also in pathophysiologic adaptation as in diabetes, hypertension, and preeclampsia. The mechanisms that regulate vascular tone are not fully understood. Moreover, whether abnormal changes in vascular tone in various vascular beds play a causal role in the pathogenesis of cardiovascular disorders is not known. In this regard, our lab studies G protein signaling mechanisms that enable resistance arteries in the uterine vascular bed to undergo physiologic adaptation that reduces vascular tone and facilitates increased utero-placental blood flow during pregnancy.
A previous study in the lab has revealed that regulation of G protein signaling by RGS2, a GAP for Gq/11 and Gi/o class G proteins, is critical for maintaining normal uterine artery myogenic tone. Mice that are null for or harboring just one copy of Rgs2 gene have increased uterine artery vascular tone and marked decrease in uterine blood flow before and during pregnancy. We are currently pursuing studies to gain complete understanding of the mechanisms that mediate uterine blood flow regulation by RGS2 in non-pregnant and pregnant states.
| Major Research Areas |
|---|
| Contractile Function, Development of Therapeutics, G protein signaling, Heart Failure/Hypertrophy, Muscle, Renal hemodynamics, Signal transduction, Vascular biology |
| Disciplines |
| Animal Models, Molecular and cellular biology, Pharmacology and Therapeutics, Physiological Phenotyping, Signal Transduction |
| Organ Systems |
| Cardiovascular System, Endocrine System, Renal System, Reproductive System |
| Diseases |
| Acute kidney injury, Cardiac Arrhythmias, Cardiomyopathies, Chronic kidney disease, Heart Failure, Hypertension, Vasculopathy |
| Major Techniques |
| Cell Culture, Cellular and Molecular Biology, Confocal Microscopy, Dietary Interventions, Echocardiography, Electrophysiology, Ex Vivo Functional Analysis, Gel Electrophoresis/Western Blots, Immunohistochemistry, In-vivo Animal Models, In-vivo Hemodynamics, Molecular Biology, Patch Clamping, Quantitative RT-PCR, RNA Isolation and Gene Expression Analysis, RT-PCR, Radiotelemetry |
| Genes |
| Eln (), Gi/o (), Gq/11 (), Gs (), RGS2 (), RGS4 (), RGS5 () |